Management of Biological Specimens in Clinical Trials
Biological Specimens occupy a very important position in clinical trials, including PK biological samples, drug resistance biological samples, biological samples involving major efficacy indicators, etc….
The management of biological samples in clinical trial centers covers pre-collection preparation, sample collection,
sample processing, sample storage, sample transportation and other processes.
Preparation before collecting biological samples
1) Whether the teacher who collects organisms has obtained authorization and received training.
Due to the special requirements of some projects, the collection of biological samples is slightly different from the usual blood drawing operations.
All authorization and training are essential, and the actual operation time must be after the authorization and training.
2) Whether the sample collection packages and other materials from the central laboratory have been received completely.
Whether the needles, collection tubes, labels and other materials provided by the central laboratory are fully received, check the received materials against the relevant “Central Laboratory Manual”.
Collection of biological samples
1) Arrange the approximate time for blood collection with the subject and the research nurse in advance.
If liver and kidney function is involved, remind the subject that they need to fast.
2) The blood collection record form needs to be filled in in time and cannot be filled in retrospectively to ensure the accuracy and logic of sample collection time.
For example, there are visits from different subjects on the same day, and the blood collection time of subject A is 9: 00-9:05,
the blood collection time for subject B is 9:08-9:11, and the blood collection time for subject C is 9:04-9:08.
If the collector in the sample collection form is a different research nurse, this The phenomenon can be explained,
but generally the blood collection is performed by the same nurse, so there will be problems with the logic of blood collection at this time.
During the blood collection process of A, B is also collecting blood, which is not in line with the routine.
Biological sample preprocessing
1) After collection, the samples should be processed according to the relevant requirements of the
“Central Laboratory Operation Manual”, such as taking an ice bath for more than half an hour after
collection or leaving them at room temperature for half an hour after collection, etc.
Operate strictly in accordance with the requirements of the operation manual
2) Centrifugation processing, according to the requirements of the operation manual, whether centrifugation at room temperature or low temperature centrifugation is required.
Low temperature centrifugation requires opening the centrifuge in advance so that it can reach the required temperature during use.
It is also necessary to record in detail the usage account of the centrifuge and related biological sample processing.
Table, pay attention to the logic of time between samples.
3) Make horizontal and vertical comparisons through different samples from the same subject and sample
processing tables from different subjects at the same time to prevent illogical sample processing times,
just like one person operating different centrifuges and centrifuge positions.
Although they are far apart, the time of starting centrifugation is almost the same.
Storage of biological samples
1) Choose -20℃ or -80℃ ultra-low temperature storage according to requirements.
Whether the refrigerator is locked by a dedicated person and whether it is shared with samples from other projects are risks that require special attention.
2) It is prohibited to use ultra-low temperature refrigerators with automatic defrosting function.
Automatic defrosting will cause the samples to freeze and thaw repeatedly, thereby damaging the
samples and making them unusable.
3) Record in detail the “Ultra-low temperature refrigerator sample entry and exit record form”,
when the samples were in and out of the warehouse, how much is left, and whether it can be matched with the actual remaining samples.
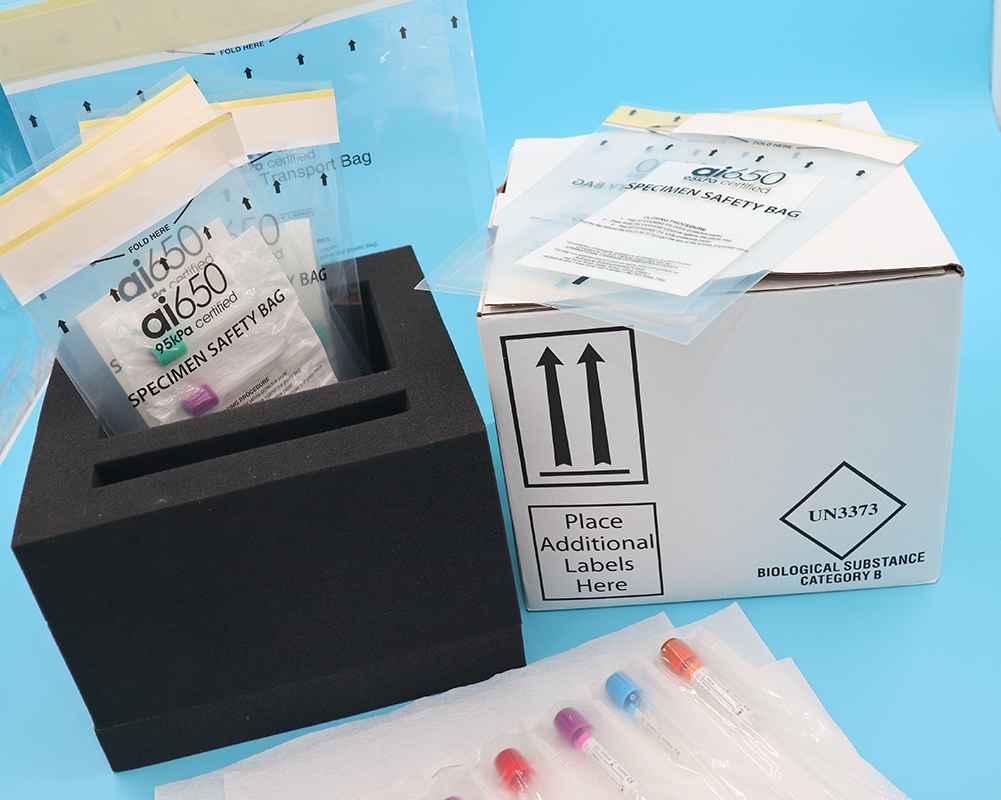
Transportation of biological samples
1) Make an appointment for cold chain logistics in advance.
Biological samples will be transported using biological logistics or urban emergency cold chain transportation. When making an appointment,
specify the quantity to be transported, the temperature to be transported, the detailed information of the sender and receiver, etc.
2) The Biological Specimens detection tube and the backup tube must be transported separately to prevent the sample from
being damaged due to temperature, external environment and other reasons during transportation,
thereby preventing normal use and resulting in the loss of data during this visit.
3) Whether the numbers in the biological sample tubes that need to be transported are recorded completely and accurately.
Check again whether the numbers that need to be transported correspond.
The number and number of samples in the “Sample Submission List” are consistent with the actual number and number transported, and Transport it to the central laboratory with the box.
After the central laboratory verifies and confirms the signature, this form, the transported thermometer calibration certificate,
the Biological Specimens transport temperature recovery and save it in the biological sample folder for verification, etc.
With the continuous development of the registration system for clinical trial institutions,
inspection of the center’s hardware and software will also be essential during future national bureau inspections, so the use, storage,
and calibration records of laboratory facilities such as ultra-low temperature refrigerators and centrifuges, etc. It may also be the focus of verification.



No responses yet