11 suggestions for transporting and storing clinical test samples
Clinical test samples
11 tips for transporting and storing
- Laboratory and sample transfer personnel should be familiar with the hospital’s sample types and quantities,
- transfer types and methods, and comply with relevant laws and regulations on sample transfer and storage.
- Transshipment personnel should be relatively stable and undergo regular training and assessment on sample transshipment knowledge.
- The laboratory should stipulate requirements for sample packaging and transportation time limits.
- When transporting infectious materials outside the hospital, the packaging and safe transportation of samples should comply with relevant national and local laws and regulations.
- The laboratory should monitor and manage the temperature, time, path, etc. during sample transportation.
- The laboratory should establish standards and procedures for sample handover and rejection, and keep records of sample handover and rejection.
- The automated logistics system should be evaluated regularly. When necessary, sample types and inspection items that are not applicable to automated logistics systems should be stipulated.
- The laboratory should specify the time limit for retaining clinical samples and the processing procedures for discarded samples.
- The laboratory should establish a closed-loop monitoring and management of sample digitization, including the application, collection, handover, computer loading, review, release, printing, destruction and other processes.
- The laboratory should develop emergency response procedures for unexpected events such as sample spillage and sample loss.
- The laboratory should integrate the management requirements for sample transportation and storage on the basis of the existing quality management system.
01
Sample classification and
Transshipment related regulations
The types of clinical test samples include blood, urine, feces, cerebrospinal serosal effusion, nasal/pharyngeal swabs, pathological tissues, etc.
Among them, blood samples are the most common, accounting for about 80%. According to the urgency of the test items, it can be divided into green channel, emergency department and ordinary clinic.
According to the hospital area of transfer, it can be divided into in-hospital transfer and out-of-hospital
transfer, and out-of-hospital transfer is further divided into inter-hospital transfer and entrusted sample transfer.
Samples that are confirmed or suspected to be highly pathogenic microorganisms (viruses) or samples
must be transported with relevant transport permits in accordance with the “Regulations on the Transport
Management of Highly Pathogenic Pathogens (Viruses) or Samples that Can Infect Humans” .
The entry and exit management of special items such as microorganisms, human tissues, biological
products, blood and their products shall be
implemented in accordance with the “Regulations on the Health and Quarantine Management of Entry-
Exit Special Items” issued by the General Administration of Quality Supervision, Inspection and Quarantine No. 160.
If the collection, preservation, and utilization of my country’s human genetic resources are provided to
external parties, the relevant provisions of
the “Regulations of the People’s Republic of China on the Management of Human Genetic Resources” must be followed.
02
Transfer personnel
Qualifications and training
In order to ensure that the transportation process does not affect the test results, is transported to the
laboratory in a timely manner, ensures safety during transportation and has the ability to initiate emergency measures in the event of an accident,
the personnel responsible for transportation should be relatively fixed and receive regular training (see training content for details) original).
Since the method and time of sample transfer are affected by various factors, which may cause errors in
the test results,
transfer personnel must undergo relevant professional training, master storage and transportation
methods, and pass corresponding assessments before they can take up their posts.
They must receive regular training every year. .
03
Packaging of samples for transport
and time limit requirements
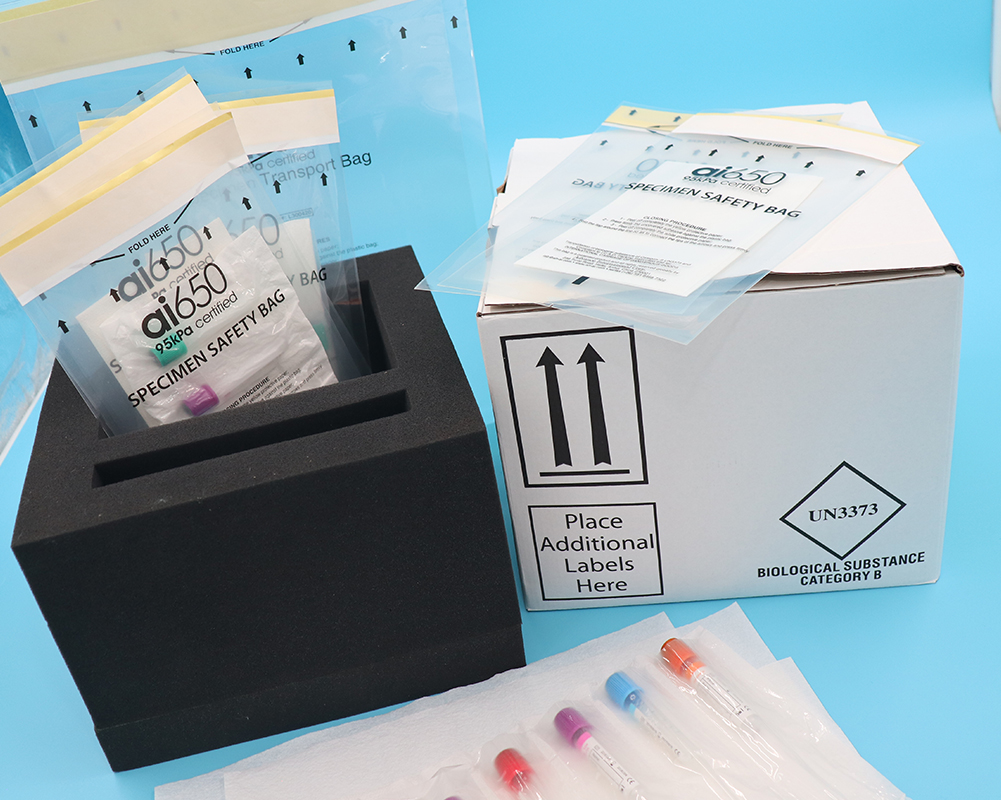
When clinical test samples are transported within the hospital,
they should be packed in sample bags with biohazard labels to prevent specimen leakage from
contaminating other samples and transport boxes.
Non-blood specimens should be packaged independently in sample bags. The sample bag is affixed with a packaging barcode label,
which displays information such as the person who packaged it, the time, and the number of samples.
The sample bag is put into a special sample transfer box.
It is advisable to use a dedicated transfer box to transfer samples to keep the temperature stable.
Appropriate fixtures or buffers should be placed in the transfer box to reduce mechanical damage during transportation,
or to buffer the impact caused by vibration and pressure changes (such as traffic accidents during transportation).
The laboratory should develop documents stipulating the specifications of sample transfer boxes, internal storage, spill prevention measures, external markings,
contact persons, etc. Different inspection items have different requirements for pretreatment methods and stabilization times.
If it is transported across a long distance, such as across hospital areas, the collector will generally perform preprocessing.
04
Transporting samples
Handover and acceptance
Before transporting the sample, the collection personnel should check the medical order again, check the sample quality,
and check the sample quantity before handing it over to the transportation personnel. The handover content includes: delivery time, delivery temperature, sample quantity, sample completeness, etc.
The handover site should meet biosafety requirements. If the sample cannot be sent for inspection in
time, the sample should be temporarily stored in suitable environment and storage conditions.
Manual transportation should monitor the environment of the transportation process in real time, such as temperature, time, path, location, etc.
In order to ensure transportation safety, the hospital implements dedicated transportation management,
sealed container transfer, fixed walking routes, strict handover registration, records handover time and personnel, and has emergency measures to prevent contamination.
Keep test tubes upright during transportation to reduce shaking and vibration, prevent spillage and
contamination, and pay attention to biological safety.
If there is a paper inspection application form, it should be delivered at the same time as the sample, and
the application form should be avoided from being contaminated.
The transport personnel must confirm whether the number of samples is consistent with the inspection
list, there is no sample leakage, and the samples are safely sent to the destination in sealed containers.
When third-party inspection agencies transfer goods across cities, they should establish a management
system to ensure the integrity and safety of transportation equipment,
including transportation methods, temperature control equipment, human resources, sample containers, protective tools, etc. When needed, drones can be used to transport samples.
The laboratory can clarify the acceptance and rejection criteria for various types of samples based on methodology and other information.
After the specimens are transported to the laboratory, they should be checked and accepted in a timely
manner, and the receipt should be confirmed after verification. The acceptance content includes:
complete application information, correct sample type, consistent sample size, timely submission for inspection, etc. Record all samples received,
including recipient and date and time of receipt. When unqualified specimens are found during acceptance, they will be recorded and processed according to the unqualified specimen management process.
05
Automated logistics
System Requirements
Common hospital logistics transmission systems include medical pneumatic logistics transmission systems, rail-type logistics transmission systems,
automatic guided vehicle transmission elevated monorail cart transmission systems, aerial transmission
systems, fully automatic intelligent blood collection tube sorting systems, etc.
Result impact assessment, biosafety assessment, quality risk assessment, and operational efficiency
assessment should be conducted on the automated logistics system (see original text for details).
Application evaluation opportunities include:
(1) When a new automatic logistics system is installed or replaced;
(2) When the location, distance, floor, etc. of the automatic logistics system are changed;
(3) The laboratory develops an evaluation cycle based on usage.
The main operator of the automated logistics system must undergo special usage training and be
authorized by the person in charge of the laboratory (see the original text for training content).
Establish an emergency plan for automatic logistics system failure, so that when the automatic logistics
system fails, it can handle it correctly or use backup methods.
06
Sample preservation
The storage of samples includes temporary storage if the collection department fails to transport them to
the testing laboratory in time, as well as storage of samples after testing. Temporarily store,
place it in a location within the visual field of employees or install monitoring to prevent samples from
being lost or forgotten;
it is recommended that the storage box be locked and can only be opened by authorized personnel;
some samples should be pre-processed or stored in the refrigerator.
The original samples after inspection should be placed in order, marked with dates and stamped, and
stored in refrigerated compartments for review and dispute appeal.
The specific storage time can be determined by the laboratory based on space, facilities, and needs.
When the laboratory detects samples that are positive for highly pathogenic microorganisms such as human immunodeficiency virus,
they should be transferred to the Center for Disease Control and Prevention where the laboratory is located for processing.
If there are no special circumstances in the test results, the tested samples should be treated as medical
waste after exceeding the prescribed storage period.
If the sample is retained for scientific research, it will be managed as a scientific research sample and
must be approved by the hospital ethics committee.
07
transport process
Tracking and Monitoring
In order to track the whereabouts of samples, it is necessary to record the number of samples, date and
time, transport personnel, and transportation method for each handover.
You can check the quantity and specific information during transportation.
Apply time node control to the circulation process to provide managers with effective circulation time monitoring and analysis data. The controllable time nodes in the laboratory process include: inspection application, sample collection,
nursing and logistics handover, inspection department reception, receipt by each professional group, on-machine testing,
test completion, result review, result release, query or print report, and sample Destroy etc.
It supports setting an early warning function in each node or for a special link to prompt transportation
limit requirements.
Once there is an emergency, real-time alarm prompts will be issued from the issuance of medical orders,
sample collection, post-collection transfer, and laboratory department reception.
Once a critical value result occurs, early warning will be issued one by one from instrument transmission,
result review, report sending, clinical result viewing and other links.
08
transport process
Accident emergency response
The transfer process should be equipped with a spill bag, which can include medical waste bags, gauze,
medical gloves, disposable tweezers, disinfectant wipes, etc.
Accidents during the transfer process mainly include sample spillage and sample loss. The laboratory should develop a blood (body) fluid spillage treatment process.
When a small amount of blood (body) fluid (<10 ml) spills, use a spill bag, take personal protection, wear gloves, and if necessary,
wear protective clothing, eye protection, and goggles. Cover the area contaminated with blood (body)
fluids with a cloth or paper towel, absorb the blood (body) fluids and put them in a yellow garbage bag.
Disinfectant wipes clean from outside to inside until blood (body) fluids are invisible to the naked eye. Change gloves.
Wipe the disinfectant wipes from outside to inside and keep them moist for 2 to 3 minutes to dry naturally.
Remove gloves, perform hand hygiene, and record handling conditions.
Unqualified samples due to sample overflow, temperature control, time delay, etc. should be truthfully
recorded and fed back in a timely manner.
09
Quality of the transfer process
Security system construction
You can refer to ISO 15189, CAP Checklist, CLSI and other standards, combined with the laboratory’s own
conditions, according to my country’s standards, specifications and guidelines,
from the organizational structure, user-centered, facilities and safety, personnel, procurement and
inventory, equipment, process management Establish a quality and safety management system for the transfer process in terms of documents and records,
information management, non-conformity event management, evaluation and continuous improvement to provide continuous guarantee for the accuracy and timeliness of inspection results.

No responses yet